QUS
Ultrafast Boosted Liver Steatosis Assessment
Quantifying steatosis in the liver is the first step in the diagnosis of MASLD and primarily uses non-invasive ultrasound. Initially visual (qualitative), the assessment of fat in the liver has benefited from the development of quantitative ultrasound (QUS) which now offers objective measurements of steatosis-related parameters, surpassing subjective visual assessments.
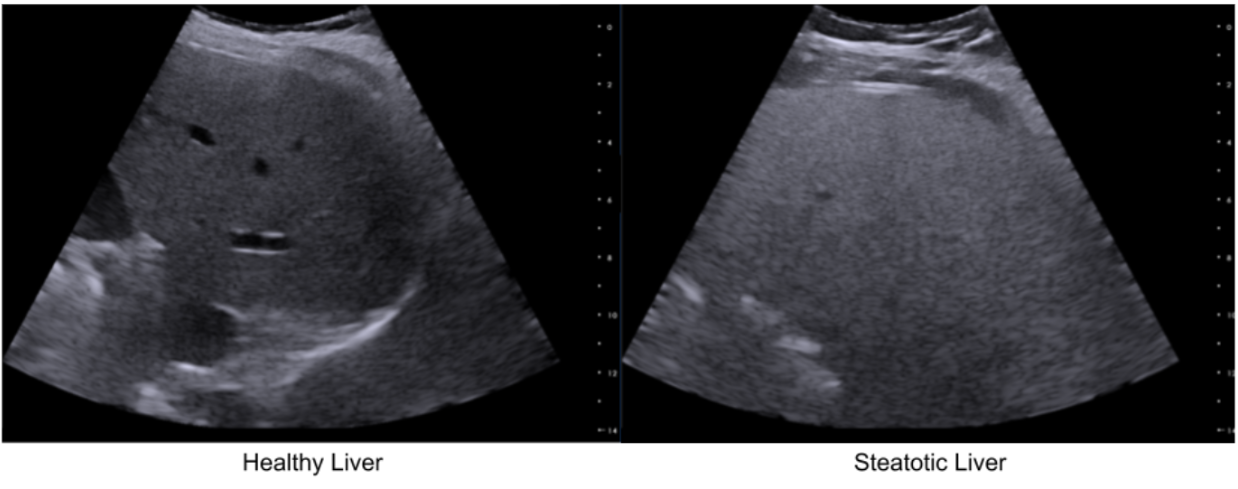
Increased intracellular fat elevates the backscattering coefficient (BSC), making the liver appear brighter (“bright liver”). Fat also causes higher attenuation (ATT), i.e. signal loss and posterior shadowing, and decreases the speed of sound (SOS) as lipid (fat) medium is a slower medium than healthy (aqueous) liver tissues.
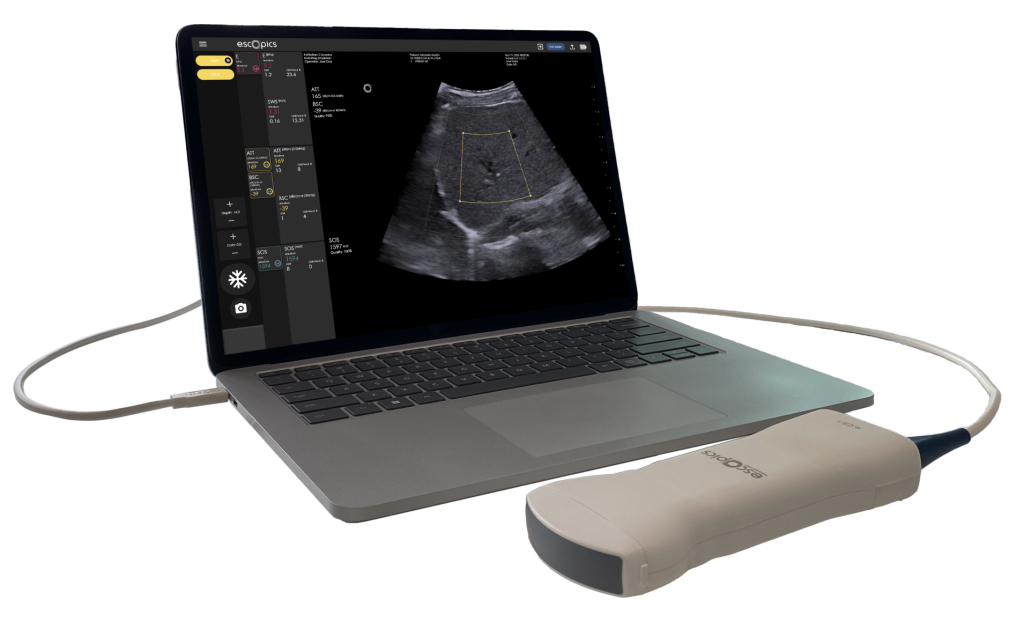
Hepatoscope, thanks to its QUS workflow, is the unique system offering real-time ATT, BSC and SOS estimation based on ultrafast ultrasound imaging. The estimation of BSC and ATT are performed using a reference-based method. This approach is powerful because it uses a well-characterized reference phantom to cancel out system-dependent variables.
By comparing signals from the tissue to the signal from the phantom, one can isolate the intrinsic acoustic properties of the tissue. Coupling ultrafast ultrasound imaging with the reference-based approach presents several benefits, including improved accuracy and robustness thanks to extensive signal averaging and compounding, mitigation of motion artifacts by “freezing” physiological movement, better statistical averaging for heterogeneous tissues, larger sampling volume in the liver, and reduced operator dependency, leading to more consistent and reliable quantitative assessments, which is crucial for monitoring fat content in the liver of patients with MASLD.
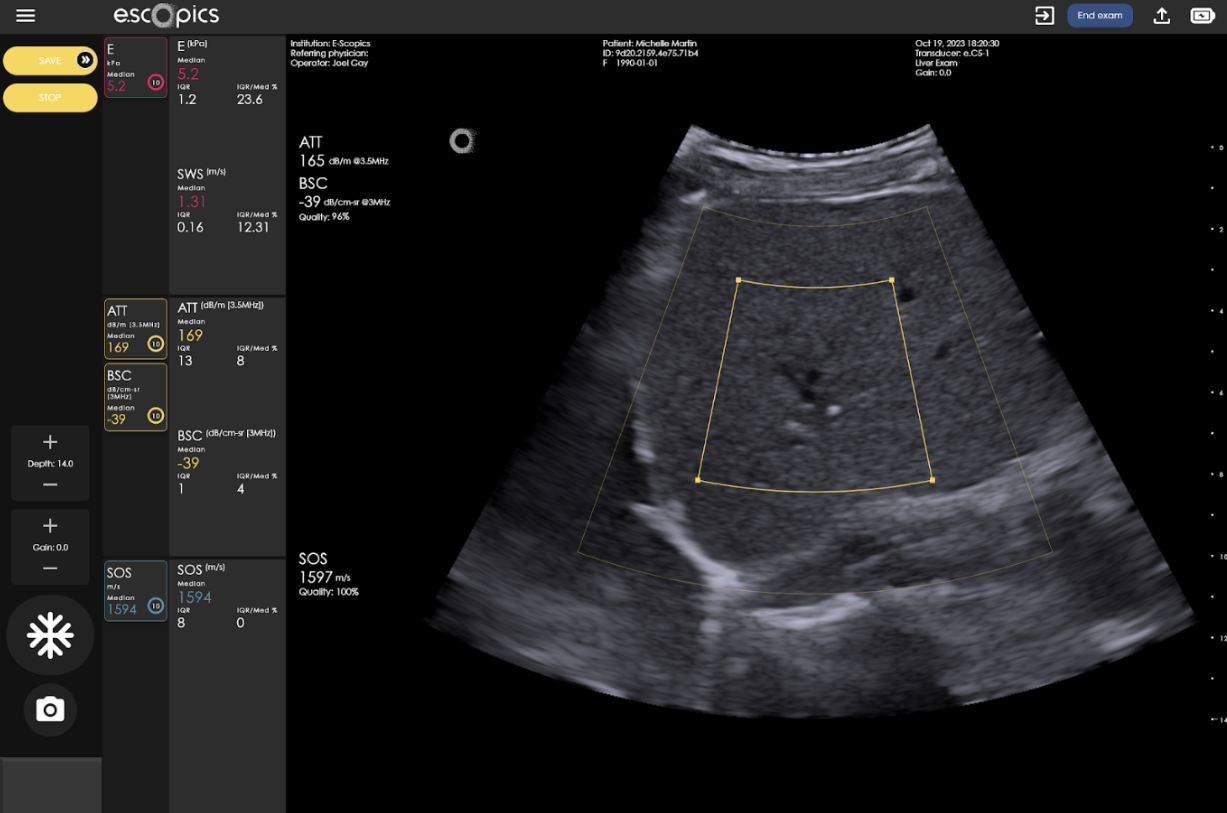
Regarding SOS estimation, Hepatoscope implements a patented method developed at E-Scopics (Heriard-Dubreuil et al. 2023). The core innovation is a novel SOS estimation technique that utilizes an angular approach based on plane waves to exploit refraction properties in layered media. This allows for the inference of local speed of sound values directly from angular raw data, offering robust, accurate, and real-time measurements with significantly fewer emissions and lower computational time compared to the current other existing methods.
Ultrafast acquisitions enable a real-time measurement of a quality index, guiding operators by quantifying acoustic signal reliability. This multifactorial index, derived from signal stability, signal-to-noise ratio, and model goodness-of-fit, indicates trustworthy measurements when high.
Clinical benefits include increased confidence and reduced error by discarding unreliable data (e.g., from rib shadows, poor probe contact, large blood vessels). Immediate visual feedback with B-mode imaging optimizes probe positioning and data acquisition, dramatically improving measurement reproducibility and reliability.
At E-Scopics, we are aware that the clinical adoption of QUS biomarkers requires measurements to be accurate and reproducible across different ultrasound systems, operators, and institutions.
To address this critical need for standardization, our team actively contributes to the QIBA PEQUS (Quantitative Imaging Biomarkers Alliance – Pulse-Echo Quantitative Ultrasound) initiative.
References
- Bercoff, Jérémy, Mickaël Tanter, and Mathias Fink. 2004. “Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping.” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 51 (4): 396–409.
- Catheline, S., F. Wu, and M. Fink. 1999. “A Solution to Diffraction Biases in Sonoelasticity: The Acoustic Impulse Technique.” The Journal of the Acoustical Society of America 105 (5): 2941–50.
- Lerner, R. M., S. R. Huang, and K. J. Parker. 1990. “‘Sonoelasticity’ Images Derived from Ultrasound Signals in Mechanically Vibrated Tissues.” Ultrasound in Medicine & Biology 16 (3): 231–39.
- Muthupillai, R., D. J. Lomas, P. J. Rossman, J. F. Greenleaf, A. Manduca, and R. L. Ehman. 1995. “Magnetic Resonance Elastography by Direct Visualization of Propagating Acoustic Strain Waves.” Science (New York, N.Y.) 269 (5232): 1854–57.
- Nightingale, K. R., M. L. Palmeri, R. W. Nightingale, and G. E. Trahey. 2001. “On the Feasibility of Remote Palpation Using Acoustic Radiation Force.” The Journal of the Acoustical Society of America 110 (1): 625–34.
- Ophir, J., I. Céspedes, H. Ponnekanti, Y. Yazdi, and X. Li. 1991. “Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues.” Ultrasonic Imaging 13 (2): 111–34.
- Tanter, Mickael, and Mathias Fink. 2014. “Ultrafast Imaging in Biomedical Ultrasound.” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 61 (1): 102–19.
- Yin, Meng, Olivier Rouvière, Kevin J. Glaser, and Richard L. Ehman. 2008. “Diffraction-Biased Shear Wave Fields Generated with Longitudinal Magnetic Resonance Elastography Drivers.” Magnetic Resonance Imaging 26 (6): 770–80.
